





Ear surgeries such as tympanoplasty for cholesteatoma, stapes surgery for otosclerosis, and facial nerve decompression for facial nerve palsy are performed by certified surgical instructor of the Japan Otological Society. Many cases of tympanic membrane perforation can be repaired with basic fibroblast growth factor (bFGF) as a day surgery. Audiologists and speech therapists provide medical care including hearing aids for patients with sensorineural hearing loss. We are also actively treating cochlear implants and implantable bone conduction devices for adult patients with profound hearing loss.
Botulinum toxin injection therapy for spasmodic dysphonia has a history of being started in our department for the first time in 1993, and up to now, more than 400 people have been treated. We also perform surgical treatment for laryngeal polyps, tumors, or vocal cord paralysis.
For children and adult patients with SDB, overnight polysomnography (PSG) and drug-induced sleep endoscopy (DISE) are performed by specialized PSG technologists, and anesthesilogists, respectively. Surgical treatments for OSA are strictly indicated. Continuous positive airway pressure (CPAP) is managed in collaboration with local clinics. A certified doctor of the Japanese Society of Sleep Research supervises these medical examinations and treatments.
We are actively performing endoscopic nasal sinus surgery or biological injection therapy that is the latest treatment method for patients with chronic rhinosinusitis. For allergic rhinitis, we offer sublingual immunotherapy or biological injection therapy as well as laser treatment for nasal mucosa.
We also provide safe and reliable treatment of head and neck tumors. We treat malignant tumors in collaboration with university hospitals or cancer centers.


Subspecialty: Otology, Sleep Medicine, Rhinology,
Pediatric Otorhinolaryngology
| Title | Professor |
|---|---|
| Name | Masaaki Suzuki M.D. Ph.D. |
| Country | Japan |
| Affiliation | Teikyo University Chiba Medical Center |
| suzukima@med.teikyo-u.ac.jp |



Subspeciality: voice, swallowing disorder, neurotology, head & neck surgery
Graduate: Graduate from Yamanashi University School of Medicine 2011
Subspeciality: rhinology, general otorhinolaryngology
Graduate: Graduate from Teikyo University School of Medicine 2017
Subspecialty: Logopedics and Phoniatrics, Laryngology
Graduate: Graduated from Teikyo University School of medicine, Tokyo, Japan in 2014
Subspeciality: rhinology, general otorhinolaryngology
Graduate: Graduate from Teikyo University School of Medicine 2011
Yasuhisa Chiba M.D.
Yuriko Irie M.D.
Sugimoto Akira M.D., Ph.D.
Takeshi Suzuki M.D., Ph.D.
Fukie Omori M.S.
Minori Aoki
Ayako Kikuchi
The pathogenesis of obstructive sleep apnea (OSA) is characterized not only by obstruction of the pharynx, but also by repeated obstruction. OSA onset is thought to involve four phenotypic traits: pharyngeal muscle responsiveness, respiratory center instability (loop gain), arousal threshold, and anatomical factors. Patients with lower muscle responsiveness are likely to have OSA, whereas those with higher responsiveness are not. When the loop gain is relatively high, reaction and suppression of the respiratory drive are repeated, decreasing ventilation and pharyngeal muscle activity and leading to mixed or central apnea events. Patients with a low arousal threshold tend to have frequent respiratory events and less severe respiratory efforts, whereas those with a high arousal threshold tend to have fewer respiratory events and more severe respiratory efforts. Pharyngeal muscle activity, as well as respiratory drive, increases during apnea and decreases after its release. Patients with a low arousal threshold have lower muscle responsiveness and instability of the respiratory center control, whereas those with a high arousal threshold have higher muscle responsiveness and relatively stable respiratory control. The overshoot and undershoot responses of the chemical drive and pharyngeal muscle tone characterize the periodic repetition of obstructive events, which are enhanced by the arousal response. The presence of certain anatomical factors is prerequisite for the onset of OSA. Also, not only volume and flow, but also stiffness and elasticity may contribute to the pathogenesis of OSA. Mouth breathing also plays an important role in the mechanism of pharyngeal collapse. These four factors influence each other, with the first three—muscle responsiveness, loop gain, and arousal threshold—in particular in a trinity. The era is already close in which not only anatomical treatment, but also treatments for other traits can be selected and combined according to the individual pathophysiological condition of each patient with OSA.
The fundamental basis of sleep apnea is that control by the respiratory center is involved in obstructive sleep apnea (OSA), whereas airway narrowing is involved in central sleep apnea. The pathogenesis of OSA is affected by various factors, which interact to form a complex pathological condition. Therefore, the apnea-hypopnea index (AHI) alone cannot fully address the pathophysiology of the disease. A model proposed by Wellman posits that OSA onset involves four phenotypic traits: pharyngeal muscle responsiveness, respiratory center instability, arousal threshold, and anatomical factors [1]. This model can determine whether a patient has OSA and its severity. These four factors influence each other.
The first step in understanding the pathophysiology of OSA is to understand the physiological phenomena read from the traces on polysomnography (PSG) (Fig. 1a). Sleep onset reduces the response to intrapharyngeal pressure, which physiologically suppresses and relaxes pharyngeal muscle activity, resulting in narrowing and collapse of the pharyngeal airway. The pharyngeal narrowing and collapse cause airflow reduction and respiratory effort as responses of the mechanical receptors on the pharyngeal wall and stretch receptors on the chest wall to the negative pressure caused by the respiratory effort. The activity of pharyngeal muscles (i.e. muscle tone) gradually increases, leading to muscle contraction. Pharyngeal muscle responsiveness refers to the contraction response that restores airflow. The muscle activity progressively increases until it reaches the opening threshold (the effective recruitment threshold) [2]. The blood gas tension deteriorates and the chemical drive increases along with respiratory effort and negative pharyngeal pressure. When the arousal threshold is below the effective recruitment threshold, arousal occurs, the airway opens, and ventilation starts again with loud snoring [2, 3]. The overshoot and undershoot responses of the chemical drive and pharyngeal muscle tone characterize the periodic repetition of obstructive events. In this review, we discuss each phenotypic trait in light of new evidence.
The pharynx has three important functions: swallowing, breathing, and phonation. The pharyngeal lumen must be completely closed for swallowing but must be open for breathing. Essential elements for phonation are elasticity and compliance, which adjust the stiffness and shape of the lumen. To perform these various complex functions, the pharynx is a collapsible tube composed of many dilator and stiffener muscles that regulate the integrated muscle activity. The tongue contains extrinsic and intrinsic muscles. Most of these muscles are under the control of the hypoglossal nerve, with the extrinsic muscles moving the tongue and the intrinsic muscles adjusting the shape. The extrinsic muscles comprise protrudors and retractors. Protrudors pull the tongue forward and retractors pull the tongue backward [4]. When the hypoglossal motor neurons are activated, the tongue contracts; otherwise, it relaxes. There are two muscle contraction patterns, phasic and tonic. In the phasic pattern, muscle activity increases during inspiration, forming a burst (strong reaction with a steep peak), and decreases during expiration. In the tonic pattern, muscle activity shows a continuous tone in both the inspiratory and expiratory phases. The genioglossal (GG) muscle is a representative protrudor with a phasic pattern that has been examined in many physiological studies of sleep-disordered breathing.
Sleep onset suppresses the control of the pharyngeal muscle. The contractive muscle tone then increases as the muscle responsiveness increases, leading to periodic repetition of apneas and hypopneas. In individuals whose arousal threshold is above the effective recruitment threshold, the pharynx opens only in response to increased pharyngeal muscle tone without arousal [5]. In this case, arousal is no longer essential for airflow restoration, often seen in individuals with a strong anatomical factor. Patients with lower muscle responsiveness are more likely to have OSA, whereas patients with higher muscle responsiveness are less likely to develop it [6].
Pharyngeal muscle responsiveness in patients with OSA is associated with (A) control by the respiratory center and (B) the contraction and sensory function of the muscle.
Three pathways are involved in pharyngeal muscle control. First, mechanoreceptors on the pharyngeal mucosa react to the negative pressure during sleep, with the pharyngeal muscle then contracting through the hypoglossal nerve nucleus of the medulla oblongata. Second, tonic muscle control is regulated by serotonin, choline, or noradrenergic neurons. Third, efferent muscle control regulated by carbon dioxide-sensitive chemoreceptors is under the control of the respiratory center. The responsiveness of the pharyngeal muscles decreases due to the loss of the two former control functions at sleep onset and during rapid eye movement (REM) sleep [7]. Numerous electrophysiological studies have investigated the response of the GG muscle by electromyography (GG-EMG). Akahoshi et al. detailed a highly reproducible and strong correlation between the negative pressure in the pharyngeal airway and the GG-EMG, which was strong even when the chemical regulatory system was stimulated by oxygen or carbon dioxide [8].
The pharyngeal muscles of patients with OSA have reduced tonic muscle control at the end of exhalation and phasic muscle control at maximal inspiratory effort compared with those in the healthy population [9]. Saboisky et al. measured the action potential of a single motor unit (a group of muscle fibers regulated by one motor nerve) of the GG and found that the duration and area of the action potential were decreased compared with healthy individuals, indicating that the pharyngeal muscles were electrophysiologically degenerated [10]. The pharyngeal muscle response during REM sleep has been found to be significantly lower than those during non-rapid eye movement (NREM) sleep and wakefulness, explaining the increases in respiratory events in REM sleep compared with NREM sleep in patients with OSA [7, 11]. Malhotra et al. reported a significant decrease in GG muscle responsiveness to experimental negative pressure during lateral sleep compared with supine sleep in patients with OSA, suggesting that pharyngeal occlusion is prevented during supine sleep when the upper airway is vulnerable to collapse [12]. Interestingly, animal experiments have suggested that the vestibular function in the inner ear may be involved in the response of the GG muscle [13]. The pharyngeal dilator and stiffener muscles contract when the apnea is released [14]. This simultaneous contraction of the dilator and stiffener muscles decreases pharyngeal wall compliance and releases a respiratory event. Therefore, care must be taken not to cut the pharyngeal muscles during sleep surgeries.
According to Wellman’s model, PSG and continuous positive airway pressure (CPAP) can be used to determine the four traits. Individuals put on a CPAP mask and a variety of pressure changes is applied. The volumetric flow rate (L/min) at each point is measured as the pressure is lowered or increased over a total measurement time of about 15 min. Four phenotypic traits can be drawn on a graph, with respiratory drive (L/min) on the horizontal axis and ventilation volume (L/min) on the vertical axis. The onset, severity, phenotype (obstructive, central, or mixed), and therapeutic efficacy of OSA can then be predicted by analyzing the interaction of these traits. Passive pharynx refers to the state in which necessary and sufficient pressure is applied during sleep, with the pressure suddenly reduced to zero when the muscle tension disappears (passive V0 point). Active pharynx refers to the state at which the patient puts on the CPAP mask, with the pressure then slowly lowered to the threshold at which muscle tension does not cause arousal [1]. The pharyngeal muscle response is expressed by the gradient of the difference between the ventilation volume in the active pharynx (Vactive) and that in the passive pharynx (Vpassive), which reflects the amount of the change on EMG between the active and passive pharynx. The balance between the pharyngeal critical closing pressure (Pcrit), which is an index of pharyngeal collapsibility [15], and the GG-EMG change amount divided by the pharyngeal pressure change amount somewhat delineates whether or not someone will develop OSA [5]. Compensation is expressed by the difference between Vpassive and Vactive and is also used as an indicator of pharyngeal muscle responsiveness. This compensation has been reported to be low in typical OSA patients with a low arousal threshold and a high AHI [16].
Histopathological studies of the degeneration of nerve endings in the pharyngeal muscles have been conducted by researchers, including otolaryngologists, since the 1990s [17,18]. Such studies showed that type I fibers that were resistant to muscle fatigue shifted to type IIA fibers that were prone to muscle fatigue in the palatal and tongue muscles of patients with OSA [19,20]. Histopathological findings from the pharyngeal muscles in patients with OSA also indicated the presence of atrophy and hypertrophy, such as the infiltration of inflammatory cells and increased connective tissue due to inflammation caused by hypoxic stress and snoring vibration [21]. Studies using monoclonal antibodies revealed prominent expression of neural cell adhesion molecule, which is an index of denervated muscle, in the pharyngeal muscles in patients with OSA, and its association with muscle degeneration and degeneration of nerve endings [22]. This damage to the muscle contraction strength is thought to be associated with decreased responsiveness of the muscle in patients with OSA [23]. The sensory perception function on the surface of the pharyngeal mucosa has also been reported to be impaired in patients with OSA. Kimoff et al. measured the perception between two points by applying an air jet to the pharyngeal surface in patients with OSA. They found deterioration of receptor function on the surface of the pharyngeal mucosa and its improvement with treatment [24]. The same group reported a correlation between the sensory perception function in the pharynx and the AHI [25]. Kim et al. compared perception between two points by directly touching the pharynx with a thin monofilament, finding that the receptor function on the surface of the pharyngeal mucosa was reduced in patients with OSA [26]. These impairments of sensory function led to a reduction in afferent feedback signal transmission from the pharynx to the respiratory center, which in turn led to decreased pharyngeal muscle responsiveness [23].
Some contemporaries in the field believe that decreased muscle contraction strength due to myopathy does not itself contribute much to the pathology of OSA because the GG-EMG activity in patients with OSA decreases when respiratory events occur, but it is well-maintained during sleep with stable breathing and is even higher than that of healthy individuals during wakefulness [27]. However, it is natural that decreases in the action potential of a single motor unit could affect the neuropathy of the pharyngeal muscle [23]. Thus, there may be a link between decreased muscle responsiveness and muscle degeneration in patients with OSA.
Respiration is subject to chemical, neural, and mechanical regulation, stimulated by the respiratory center on the ventral side of the medulla oblongata via the efferent nerve and fed back to the respiratory center via receptors such as partial pressure of carbon dioxide (PCO2) or the vagus nerve. This closed system formed by respiration is called a “loop”. Respiratory drive and ventilation are suppressed by sleep onset and respiration is mainly controlled by chemical regulation during NREM sleep to keep the PCO2 constant.
One of the established methods for investigating the chemical ventilation response in the respiratory center is Read’s re-breathing method. The subject inhales and exhales in a closed circuit, which gradually increases the carbon dioxide concentration. The change in ventilation volume is then measured and graphed. Carbon dioxide stimulates ventilation primarily through the central chemoreceptor on the ventral side of the medulla oblongata. We previously reported significant differences in the hypercapnic ventilatory response measured with Read’s re-breathing method between surgical responders and poor responders among younger non-obese, normocapnic, normal craniofacial patients with OSA: this was the first time that the ventilatory response was shown to influence the effectiveness of upper airway surgery in patients with OSA [28].
On the other hand, according to Wellman’s method, respiratory center instability (loop gain) is measured based on a single change in ventilation volume under CPAP by dial-up only once from active pharynx to passive pharynx [1]. The magnitude of the ventilatory response to change is explained using the concept of loop gain, which is a bioengineering theory. Loop gain is the value obtained by dividing the response (ventilation response) by the dynamic modulation (ventilation change amount) when some dynamic modulation is applied to the system. A high loop gain induces a large response to changes in ventilation, whereas a small loop gain induces a small response. Loop gain is physiologically explained by controller gain, which is the respiratory output, and plant gain, which is the change in chemical reactivity. These can be plotted with carbon dioxide tension on the horizontal axis and alveolar ventilation volume on the vertical axis [29]. In individuals with OSA who have impaired respiratory chemosensitivity (high loop gain), the ventilatory response changes markedly, making it easier to repeat the overshoot and undershoot and resulting in central sleep apnea. If the ventilation exceeds the level required due to overshoot stimulation, ventilatory control decreases the respiratory drive and PCO2 below the apnea threshold, leading to central apnea. We previously reported a case of multiple system atrophy that initially manifested as primarily obstructive components; however, central sleep apnea predominantly appeared after removing the upper airway obstruction with CPAP treatment [30]. In contrast, in individuals with a relatively high loop gain, reaction and suppression are repeated and the ventilation drive and the muscle activity decreased, leading to airway narrowing. However, in these individuals, respiratory events are likely to be obstructive with attenuated chest and abdominal movements. A periodically repeated obstructive respiratory cycle is a manifestation of unstable ventilatory control [2]. However, the extent of the involvement of PCO2 and partial pressure of oxygen (PO2) in this cycle might depend on the individual phenotype.
Arousal is caused not only by individual characteristics and external stimuli such as nasal obstruction or acid reflux [31, 32], but also by output from the respiratory center. Arousal due to increased respiratory output occurs even when it is associated with respiratory events such as an increase in PCO2 or a decrease in PO2, which varies considerably among individuals and sleep stages.
We previously reported that the arousal threshold is correlated with OSA severity [33]. Because the arousal threshold increases with increased OSA severity, individuals with better anatomy should be more likely to have a non-anatomic deficiency causing OSA, such as a low arousal threshold. An individual with a low arousal threshold wakes up before a severe gas exchange abnormality (low SpO2) has developed. In these cases, arousal occurs earlier during increased muscle tone before the effective recruitment threshold, leading to lower muscle responsiveness. Repeated arousals could promote dynamic ventilatory instability [34] and subsequent respiratory events. Therefore, the AHI tends to be high. Repeated arousals also may prevent the achievement of a slow wave, leading to worse sleep quality [2]. On the other hand, individuals with a high arousal threshold are resistant to arousals and tend to have fewer respiratory events but have more severe respiratory efforts that are longer and have a lower minimum oxygen saturation. Edwards et al. used an epiglottic catheter during overnight PSG to measure the nadir epiglottic pressure before arousal as the arousal threshold. The results showed a negative correlation between the minimum oxygen saturation and the arousal threshold, suggesting that patients with a higher arousal threshold had a lower minimum oxygen saturation [35]. Younes demonstrated that arousals promote a greater overshoot of the ventilated drive as well as a greater overshoot of the chemical drive [5].
The odds ratio product (ORP) is considered to reveal new insight into the arousal threshold derived from the relationships of the EEG power spectrum (delta, theta, alpha, sigma, and beta waves) in different frequencies to each other [36]. The ORP is a continuous metric ranging from 2.5 (full wakefulness) to 0 (very deep sleep) that decreases progressively as the EEG transitions from full wakefulness to sleep onset and also from light to deep sleep stages. Considerable evidence supports its use as an index of sleep depth and intensity. More importantly, the correlation between the ORP in any given 30-s epoch and the probability of a spontaneous cortical arousal or awakening occurring within the next 30 s (i.e. the arousal threshold) is almost perfect [36].
Physiologically, an anatomical indicator of upper airway collapsibility has been established by the objective intratracheal pressure when the pharynx is occluded, namely, Pcrit [15]. The methods used in that study involved transient reductions in the CPAP delivered to the mask (Pmask) during stable non-REM sleep for five breaths and the delivery of multiple reductions in CPAP throughout the night; the relationship between the Pmask and peak inspiratory flow was then plotted. The point at which the linear regression crossed the x-axis was the Pcrit. The higher the Pcrit value, the higher the OSA severity, with most normal individuals without OSA showing a negative range. Wellman simply defined the anatomical factor as the ventilation volume at the passive V0 point (the point at which the CPAP pressure suddenly decreases to zero under passive pharynx conditions) [1]. As a model to explain the anatomical factors in patients with OSA, the anatomical balance model from Japan has gained consensus worldwide [37]. The theory is that the cross-sectional area of the pharyngeal airway is determined by the balance between the bony structure comprising the maxillary and mandibular bones and vertebrae and the soft tissues comprising the tonsils, tongue, pharyngeal lateral walls, and soft palate contained therein. Pharyngeal narrowing occurs because of an imbalance between the soft tissue volume and the bony enclosure size. When the model loses this balance, the area of the pharyngeal airway becomes narrow, resulting in patients with OSA being unable to maintain a patent pharyngeal airway.
Our early two-dimensional analysis using cephalometry and dynamic magnetic resonance imaging (MRI) revealed that the features of retrognathia, micrognathia, and skeletal class II tendency were most pronounced in a group with obstruction at the retropalatal and retroglossal region and somewhat less pronounced in a group with obstruction at the retropalatal region [38]. The tendency for a long face predominated in the tonsillar hypertrophy group, whereas the presence of a long and large soft palate was highly pronounced in the retropalatal region group. All of the groups shared the characteristics of an inferiorly positioned hyoid bone compared with the control group. Another of our studies showed that patients with OSA had a wider mandibular divergence, smaller mandibular internal length, and smaller area at the mandibular base plane than controls [39]. Increased upper airway length is also associated with greater susceptibility to airway narrowing and collapse. The lung volume dependence of pharyngeal airway collapsibility is evident in anesthetized and paralyzed patients with OSA [40]. An increased lung volume structurally improves velopharyngeal collapsibility. A positive co-relationship between body mass index (BMI) and Pcrit at the velopharynx has been observed.
Although the mechanism of pharyngeal collapse has often been explained on the basis of the two-dimensional pharyngeal cross-sectional area, the details of the pathogenesis of OSA could be clarified by three-dimensional (3D) structural analyses. We reconstructed a 3D MRI model of pharyngeal soft tissues and craniofacial structures and then calculated the 3D pharyngeal anatomical balance by dividing the sum of the soft tissue volume by the craniofacial volume [41]. Patients with OSA had a smaller craniofacial volume and larger pharyngeal soft tissue volume compared with control subjects, resulting in a significantly higher 3D pharyngeal anatomical balance than in controls. There were significant differences in the 3D pharyngeal anatomical balance between OSA and control. We also revealed that positional OSA had a relatively small total pharyngeal soft tissue volume and the smallest craniofacial volume, whereas nonpositional OSA had a relatively large craniofacial volume and the largest total pharyngeal soft tissue volume. There were no significant differences in the 3D pharyngeal airway anatomical balance between the positional and nonpositional OSA patients.
The pathogenesis of OSA could be explained by size and volume in a 3D reconstructed model; however, recent studies have shown that not only size and volume, but also stiffness can contribute to the pathogenesis of OSA. Kim et al. suggested that the tongue was significantly larger and had an increased amount of fat in people with apnea than in controls after adjustment for age, BMI, sex, and race [42]. They also showed regional differences in fat distribution, with increased tongue volume and deposition of fat at the retroglossal region in obese patients with OSA compared with obese controls. Tongue fat volume was correlated with AHI and BMI, and the amount of tongue fat in obese individuals with OSA is greater than that in those without OSA. Brown et al., using magnetic resonance elastography, showed that tongue stiffness (quantified as the shear modulus) was lower in patients with OSA than in age- and BMI-matched controls [43]. The anisotropic data suggested that the decrease in tongue shear modulus in OSA occurred in the direction of the muscle fibers. The viscoelastic properties of the pharyngeal airway were also found to influence its shape and size. Their investigations indicated that the stiffness of the tongue is lower in patients with OSA than in controls.
Breathing route plays an important role in the mechanism of pharyngeal collapse. Airflow in the tube is basically explained by the Starling resistor model based on Bernoulli's principle: the narrower the cross-sectional area of the stenosis, the faster the flow velocity and the higher the negative pressure. However, this model alone cannot explain the pharyngeal collapse because mouth breathing is performed. Mouth opening and oral breathing during sleep are thought to be associated with narrowing of the pharyngeal lumen and with retroglossal dimension decreases due to retraction of the tongue, which increase upper airway collapsibility and lead to airway obstruction. The open mouth position also facilitates separation of the soft palate and tongue, detachment of the posterior oral sealing, retropositioning of the mandible, narrowing of the retropalatal and retroglossal areas, shortening of the length of the pharynx, and lengthening of the mandible and hyoid distance. Meurice et al. demonstrated that mouth opening increased upper airway collapsibility during sleep [44]. Fitzpatrick et al. confirmed that, during sleep, upper airway resistance was 2.5 times higher during oral breathing than during nasal breathing [45]. Ayuse et al. examined the Pcrit in closed mouths, moderately opened mouths, and maximally opened mouths during sedation and suggested that maximal mouth opening increases upper airway collapsibility, which contributes to upper airway obstruction [46].
The physiology of the upper and lower airways and respiratory control during sleep encourage nasal rather than oral breathing. However, in nasal diseases such as septum deviation or inferior turbinate hypertrophy, the nasal obstruction can be bypassed by opening the mouth and allowing a greater volume of air to be inspired and expired. McLean et al. showed that oral breathing during sleep is induced by increased nasal resistance [47]. Patients with sleep apnea have a lower EMG tone with oral breathing than with nasal breathing, indicating pharyngeal collapse with an open mouth, whereas individuals without sleep apnea have a higher EMG tone [48]. Upper airway collapsibility and resistance during sleep have been reported to be significantly higher in people who breathe through the mouth than in those who breathe through the nose, which is different from what is seen in the conscious state. A significant increase in nasal resistance generates a higher fraction of oral breathing, leading to an unstable airway. According to our clinical data, among patients who underwent nasal surgery due to nasal obstruction, we had more patients with OSA than we expected: 10.1% of patients had a respiratory event index (REI) of 40 or higher, 16.5% had a REI of 30 or higher, and 27.8% had a REI of 20 or higher (n=79). However, most of them reported no OSA symptoms such as witnessed apnea or excessive daytime sleepiness.
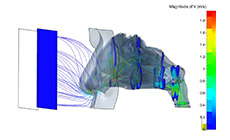
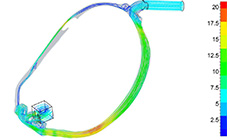
We divided oral flow (OF) during sleep into three main patterns: OF after a respiratory event, OF during a respiratory event, and spontaneous arousal-related OF [31]. Spontaneous arousal-related OF was associated with nasal obstruction, typically seen in patients with mild OSA or in controls, whereas the other two OF patterns were associated with respiratory events, typically seen in patients with moderate or severe OSA. Increased nasal resistance leads to mouth opening and oral breathing. If nasal airway obstruction is severe with high inspiratory resistive loads, the nasal resistance exceeds a certain threshold, inducing spontaneous arousal, and nasal breathing switches to oral breathing to bypass the nasal airway obstruction. We also performed computational fluid dynamics (CFD) analyses of the nose and pharynx during nasal breathing with closed mouth, nasal breathing with open mouth, and oral breathing [49]. The results showed that airflow velocity and static pressure peaked during oral breathing, suggesting that, of the three conditions, oral breathing is the primary condition leading to pharyngeal collapse. We also showed that the airflow during nasal breathing with closed mouth was smooth throughout the whole breathing route—without spreading, perturbations, or instability—whereas that during nasal breathing with open mouth showed spreading and a disturbed, unsteady stream [49].
On the basis of these findings, we concluded that measurement of OF should be included in overnight PSG, especially in patients with habitual oral breathing.
There are two kinds of airway assessments, static and dynamic. CT and MRI imaging are static assessments, while CFD and drug-induced sleep endoscopy (DISE) are dynamic assessments. Although the upper airway has complex aerodynamics due to geometry and wall conditions, CFD technology offers an accurate and highly graphical model to understand the nature of such aerodynamics [49, 50]. The first step in CFD is analysis of velocity, static pressure, and wall shear stress according to Bernoulli’s principle. The CFD methodology was developed to evaluate upper airway airflow dynamics and has improved our understanding of pathogenesis in terms of airflow and its implications for upper airway physiology in patients with OSA. DISE examination, on the other hand, is performed to examine the obstructive site by direct observation of pharyngeal collapse during sleep. The primary advantage of DISE is its ability to visualize the airway in three dimensions. However, a major limitation is that it may cause a change in the obstructive pattern. For example, when we are assessing the oropharynx, the scope may affect the obstruction at the velum. In contrast, CFD can evaluate the airway from any angle. When we compared CFD and DISE, we found that both had consistent results and that they compensated for each other’s deficiencies. The combination of DISE and CFD would be more helpful to examine the obstructive site, obstructive pattern, and collapsibility.
The application of fluid-structure interaction simulations is considered to be a promising avenue for CFD research because these simulations can estimate the upper airway biomechanics, including stiffness, elasticity, temperature, humidity, viscosity, and flutter. Although tissue compliance is not thought to be incorporated specifically in the aerodynamics modeling, CFD would be a very effective tool if post-therapeutic static pressure could be predicted accurately in a short time because internal pressure is essential for the upper airway physiology in OSA. With more research on the interplay between fluid dynamics and further computational technological advances, surgeons could perform an in-office CFD analysis to create a personalized surgical plan for each patient that would allow for the best surgical outcome. Upper airway direct observation and physiological compensation may lead to novel methods for assessing the pathogenesis of OSA and ultimately result in more predictive surgical outcomes.
Anatomical factors such as pharyngeal tonsil (adenoid) and palatine tonsil enlargement are important in children with OSA. However, OSA is a multifactorial disorder and is not simply the result of upper airway anatomical factors, especially in small children with complications. Respiratory control is immature in infancy, and it becomes unstable and pharyngeal muscle responsiveness drops in children with congenital diseases. The degree to which the four phenotypic traits vary is diverse and differs according to the child and their age and whether they have congenital complications or not.
Wellman’s model is epoch-defining but the methodology required for precise measurements is highly complicated. For example, the model must include a system of the diaphragmatic myoelectric potential via the esophagus. The challenge for the future is to simplify the model as much as possible to enable the measurement of phenotypes in clinical practice.
The era has already arrived in which not only anatomical treatment, but also treatments for muscle responsiveness, central respiratory instability, and arousal threshold can be selected and combined according to the individual pathophysiological condition of each patient with OSA. Promising drug therapies are just around the corner. More detail could be obtained on phenotypes by subgrouping using cluster analysis. These phenotypes may also be a reflection of the pathophysiological or genetic factors, that is, endotypes or genotypes. Further analyses by factors that configure the pathogenesis of OSA or by genes and race should be developed, enabling more precise genotyping of individual patients. The era of genotype-based personalized medicine may soon be upon us.